Click to see full Important Safety Information & Prescribing Information
Indication
LUCEMYRA is indicated for mitigation of opioid withdrawal symptoms to facilitate abrupt opioid discontinuation in adults.
Important Safety Information
LUCEMYRA may cause hypotension, bradycardia, and syncope. Avoid using LUCEMYRA in patients with severe coronary insufficiency, recent myocardial infarction, cerebrovascular disease, chronic renal failure, or marked bradycardia. LUCEMYRA should be used with caution with any medications that decrease pulse or blood pressure to avoid the risk of excessive bradycardia and hypotension. Patients using LUCEMYRA should be monitored for symptoms related to bradycardia and orthostasis.
LUCEMYRA prolongs the QT interval and should be avoided in patients with congenital long QT syndrome. Monitor ECG in patients using LUCEMYRA who have renal or hepatic impairment, known QT prolongation, metabolic disturbances, pre-existing cardiovascular disease, relevant family history, or those taking drugs known to prolong the QT interval.
LUCEMYRA potentiates the depressant effects of benzodiazepines and may potentiate the CNS depressant effects of alcohol, barbiturates, and other sedating drugs.
During and after opioid discontinuation, patients are at an increased risk of fatal overdose should they resume opioid use; patients and caregivers should be informed of this increased risk. In patients with opioid use disorder, LUCEMYRA should be used in conjunction with a comprehensive treatment program.
LUCEMYRA treatment should be discontinued with gradual dose reduction.
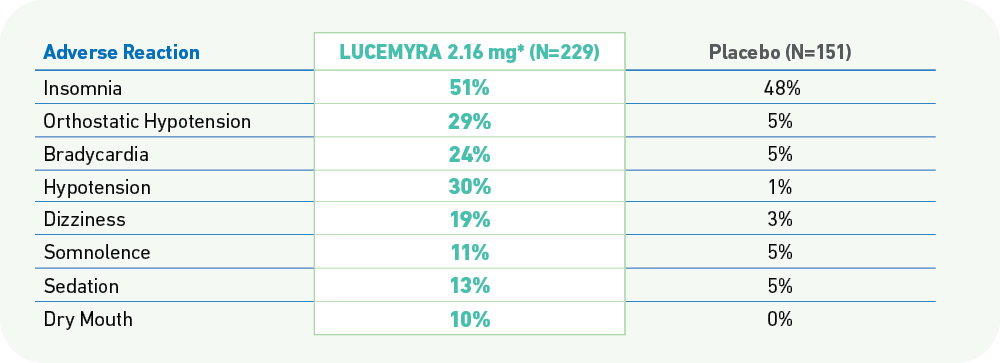
The most commonly reported adverse reactions associated with LUCEMYRA treatment (incidence ≥10% and notably more frequent than placebo) are orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.
Dose adjustment of LUCEMYRA is required in patients with hepatic or renal impairment. Before prescribing, see dosage recommendation tables in Full Prescribing Information.
There are no contraindications for taking LUCEMYRA.
To report SUSPECTED ADVERSE REACTIONS or product complaints, contact US WorldMeds at 1-833-LUCEMYRA. You may also report SUSPECTED ADVERSE REACTIONS to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information and Patient Information.